 Eight days ago the FDA approved Merck’s new Gardasil 9-valent vaccine. Great news indeed for broader protection against HPV related cancers.
Eight days ago the FDA approved Merck’s new Gardasil 9-valent vaccine. Great news indeed for broader protection against HPV related cancers.
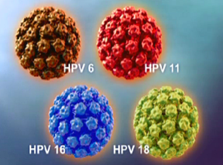
Gardasil 4-valent covers HPV types 6, 11, 16 & 18. Gardasil 9-valent covers these as well and 5 additional HPV types 31,33, 45, 52 & 58. It is interesting to note the age indication difference for males and females 9-15 and 9-26 respectively. There is roughly a 17 year window to vaccinate females but only a 5 year window for boys. As always, refer to the full prescribing information for proper vaccine comprehension and use http://www.merck.com/product/usa/pi_circulars/g/gardasil_9/gardasil_9_pi.pdf
It will be interesting to see what the recommendations will be for this new HPV vaccine, particularly for those already fully vaccinated with the Gardasil 4-valent. The ACIP will have a busy agenda for their February meeting. We at MPPG are definitely looking forward to hearing the results of their discussions and their recommendations not only for Gardasil 9, but also for the new Meningitis b vaccine Trumenba. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426485.htm
